PHD2024-22
Cilia-powered 3D flow patterns on Xenopus embryo
Host laboratory and collaborators
Julien Favier, M2P2 Julien.Favier@univ-amu.fr
Laurent Kodjabachian, IBDM laurent.kodjabachian@univ-amu.fr
Etienne Loiseau, CINAM etienne.LOISEAU@univ-amu.fr
Abstract
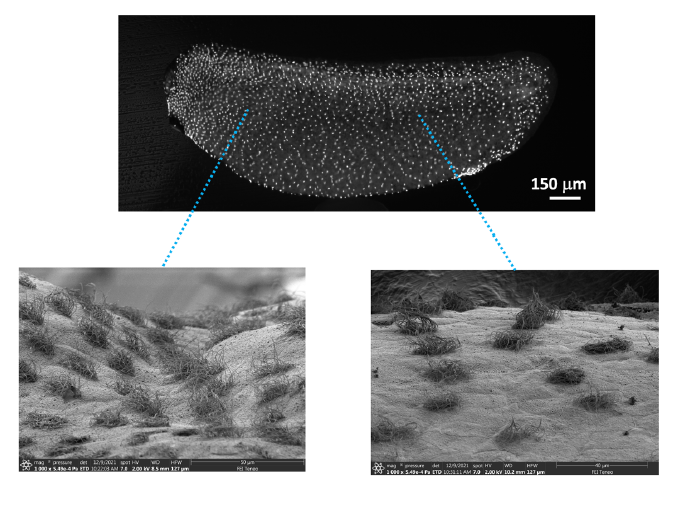
The purpose of this PhD project is to progress on the understanding of cilia-driven fluid flow in vivo, by bringing together biology, physics and numerical simulation of fluid mechanics to understand how flows powered by beating cilia are organized to protect the surface of the amphibian Xenopus embryo. This animal model has generated extensive knowledge on fundamental parameters of ciliated epithelium biology, such as ciliated cell determination, cilia synthesis, and polarized beating. In the scope of a previous Centuri PhD, a model of infection mechanisms was proposed, which shed new light on the understanding of the biological function of cilia in the Xenopus embryo. We now want to extend and test the validity of these findings to a more complex three-dimensional geometry capturing the curvature of the body and a deeper modeling of infection mechanisms by considering the active motion of bacteria. A computational fluid dynamics solver will be extended to 3D geometries including active bacteria motions, and light-sheet microscopy will be used to derive a global map of ciliated cell location and orientation, with a special focus on regions of complex topology (head, branchial arches, anal cavity), which deviate from the typical and simpler 2D organization studied so far.
Keywords
Mucociliary epithelium, cilia, cell and tissue polarity, lattice Boltzmann method, live imaging, image processing, flow patterns, collective motion, active matter
Objectives
The global objective is to build a 3D model of ciliated epithelium organization and activity, combining quantitative experimental analysis and numerical simulation of fluid transport. The ambition is to generate a multi-parametric model able to explain the emergence of cilia-driven fluid flows at the level of an entire organism, using imaging of cilia on the 3D embryo, and predictive numerical simulations for the transport of fluid, and motion of active bacteria.
Proposed approach (experimental / theoretical / computational)
- Numerical simulation (team 1): A predictive numerical model of active swimmers (bacteria) around a full 3D embryo will be built using a computational fluid dynamics solver (based on lattice-Boltzmann method). The geometry, cilia and bacteria properties will be carefully set according to quantitative experimental parameters, with the aim of generating numerical paradigms to explain the emergence of metachronal ciliary beat waves and 3D flow patterns.
- Imaging (teams 2 and 3): The PhD student will use light-sheet microscopy to record the position and orientation of all ciliated cells of the Xenopus embryo at various developmental stages. Attempts will be made to record ciliary beating and fluorescent particle movement in transgenic embryos harboring fluorescent cilia, which can be produced in team 2. Image processing methods developed in team 3 will be applied to extract quantitative information regarding cilia beating frequency and orientation, so as to build a global map of the embryo.
- Validation (teams 1, 2 and 3): In silico challenges (tissue curvature, changes in ciliated cell density or orientation) will be introduced to predict outcomes on flow patterns, which will be verified through our experimental pipeline.
Interdisciplinarity
We propose an interdisciplinary collaboration between three CenTuri groups already involved in the analysis of cilia-driven fluid flows, who have already engaged multiple collaborations, co-authored several publications and already worked together in the framework of a previous Centuri Phd project. The biologists at IBDM (team 2) have developed multiple axes of research and methods to decipher the molecular mechanisms of multiciliated cell differentiation, spatial distribution and polarity in the Xenopus embryonic skin. The experimental biophysicists at CINaM (team 3) are experts in bioactive matter, having a long-lasting collaboration with lung specialists in Marseille. They have been exploring the relationship between self-organization of ciliary activity and mucus transport for several years. The numericists at M2P2 (team 1) have developed a pioneering approach, which allowed to tackle numerically the fluid-structure interaction mechanisms governing the dynamics of ciliated cells, both at the cell level and collectively. The project draws its originality from a mixed experimental and numerical approach on a topic whose state-of-the-art mainly presents disciplinary studies.
Expected profile
The selected PhD student must have a keen interest in interdisciplinary and quantitative approaches to study biological problems. She/he will have a background in fluid mechanics and computational fluid dynamics. A taste for imaging and engineering, as well as for image processing will be an asset.
Is this project the continuation of an existing project or an entirely new one? In the case of an existing project, please explain the links between the two projects
This project is the continuation of a successful Centuri PhD project carried out by Athullya BABY entitled "Quantitative analysis and simulation of fluid flows powered by cilia in vivo". In her work, Athullya studied cilia-powered fluid flow on Xenopus epidermal explants in 2D, and our aim is now to extend her results to more realistic conditions considering threedimensional curvature of the embryo to analyse the hydrodynamical mechanisms of infections by active bacteria.
2 to 5 references related to the project
- Lattice Boltzmann simulation of creeping generalized Newtonian flows: theory and guidelines. Gsell, S., D’Ortona & Favier, J. Journal of Computational Physics, Vol. 429, 109943, 2021.
- Transport and mixing induced by beating cilia in human airways Chateau, S., D’Ortona, U., Poncet, S. & Favier, J. Frontiers in Physiology, Vol. 9, 161, pp. 1-16, 2018.
- Boutin, C. and Kodjabachian, L. Biology of multiciliated cells. Curr. Opin. Genet. Dev. 56, 1-7, 2019.
- Lrrcc1 and Ccdc61 are conserved effectors of multiciliated cell function. Aude Nommick, Camille Boutin, Olivier Rosnet, Claire Schirmer, Elsa Bazellières, Virginie Thomé, Etienne Loiseau, Annie Viallat, Laurent Kodjabachian. Journal of Cell Science, Vol. 135, N. 4, 2022.
Two main publications from each PI over the last 5 years
- Hydrodynamic model of directional ciliary-beat organization in human airways. Gsell, S., Loiseau, E., D’Ortona, U. Viallat, A. & Favier, J. Scientific Reports, Vol. 10, 8405, 2020.
- Generalized-Newtonian fluid transport by an instability-driven cilium. Wang, C., Gsell, S., D’Ortona & Favier, J. Journal of Fluid Mechanics, Vol. 965, A6, 2023.
- Nommick, A., Boutin, C., Rosnet, O., Schirmer, C., Bazellieres, E., Thome, V., Loiseau, E., Viallat, A. and Kodjabachian, L. 2022. Lrrcc1 and Ccdc61 are conserved effectors of multiciliated cell function. J. CELL SCIENCE. 135(4):jcs258960.
- Chuyen, A., Rulquin, C., Daian, F., Thome, V., Clement, R., Kodjabachian, L*$. and Pasini, A*. 2021. The Scf/Kit pathway implements self-organized epithelial patterning. Developmental Cell. 56:795-810. *Corresponding authors, $Lead contact.
- Active mucus-cilia hydrodynamic coupling drives the self-organisation of model human bronchial epithelium. Loiseau, E., Gsell, S., Nommick, A., Jomard, C., Gras, D., Chanez, P., D’Ortona, U., Kodjabachian, L., Favier, J. & Viallat, A. Nature Physics, Vol. 16, 1158–1164, 2020.
- Mesdjian, O., Wang, C., Gsell, S., D’Ortona, Favier, J., U., Viallat, A., Loiseau, E. 2022. Transition from longitudinal to transverse ciliary metachronal waves in reconstituted human bronchial epithelium. PHYSICAL REVIEW LETTERS, Vol. 129 (3), 038101, 2022
Project's illustrating image