PHD2024-09
Focused ultrasound neurostimulation for chronic pain treatment
Host laboratory and collaborators
Emilie FRANCESCHINI, Laboratoire de Mécanique et d’Acoustique LMA, emilie.franceschini@univ-amu.fr
Aziz MOQRICH, Institut de Biologie du développement IBDM, aziz.moqrich@univ-amu.fr
Thomas CHAIGNE, Institut FRESNEL, thomas.chaigne@fresnel.fr
Abstract
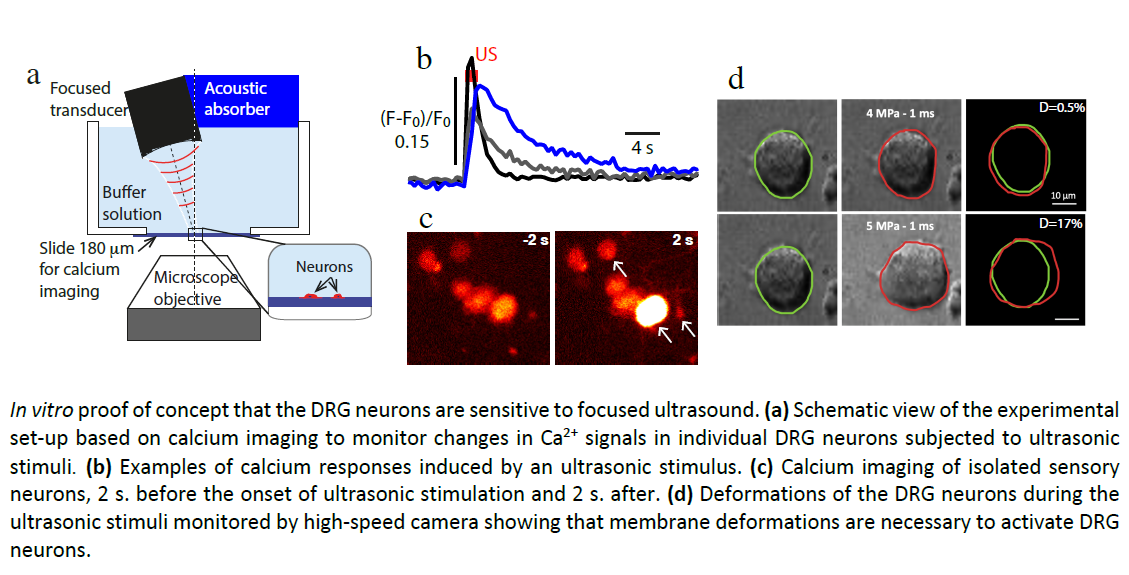
Focused ultrasound (US) neurostimulation is a promising alternative technique for chronic pain treatment. The advantages of US neurostimulation compared to standard electrical stimulation are non-invasiveness, deep penetration and high spatiotemporal accuracy. Our recent in vitro study in mouse dorsal root ganglia (DRG) neurons demonstrated that a specific class of mechanoreceptors (termed the low-threshold Cmechanoreceptors C-LTMRs) is potently excited by ultrasound than any other class. Interestingly, the C-LTMRs have been identified as strong modulators of injury-induced pain, suggesting that this particular class of neurons is likely to constitute an important, yet unexplored, target for the treatment of chronic pathological pain. The aim of this interdisciplinary PhD project is to explore the US-induced stimulation of C-LTMRs in mice in vivo and to identify the sensory pathways triggered by US stimuli using optical imaging techniques (multi-photo microscopy and/or photoacoustic tomography) in combination with molecular readouts all along the pain pathway. This will pave the way to a new generation of therapeutic ultrasound device for pain treatment.
Keywords
Ultrasound neurostimulation, chronic pain, in vivo neuroimaging, cell lineage, FOS-TRAP mice, RNA-sequencing
Objectives
The first objective of the Centuri PhD project is to explore the US-induced stimulation of C-LTMRs in mice in vivo.
Following our previous in vitro demonstration that C-LTMRs are sensitive to US stimulation, we propose to stimulate skin in mice in vivo and identify the sensory pathways triggered by US stimuli using optical imaging techniques (multi-photon microscopy and/or photoacoustic tomography). The second objective will be to understand the physical mechanisms by which US activate C-LTMRs by developing an in vitro device for measuring the effects of focused US on 3D neuronal cell models.
Proposed approach (experimental / theoretical / computational)
The mechanosensory DRG neurons that innervate the skin transmit sensory information to a complex network of neurons in the dorsal horn of the spinal cord, where this information is processed. Then this information is relayed to the somatosensory cortex for integration and perception. The skin of FOS-TRAP mouse (available at IBDM) will be sonicated by focused US. The US-activated circuit will first be identified thanks to the genetic marking of the trapped neurons at the DRGs, spinal cord and brain levels. We will then investigate several combinations of US parameters in vivo to identify efficient and reliable US protocol to stimulate DRGs (and peculiarly targeted C-LTMRs) by using optical neuroimaging techniques. Finally, the molecular and functional
identities of the trapped neurons will be unraveled using FACS sorting followed by single cell RNA-sequencing. The physical mechanisms will be quantified by conducting in vitro experiments on 3D neuronal cell models to assess the deformation of cells/nerves due to acoustic radiation force during US stimulation.
Interdisciplinarity
This project will combine the strengths of three Centuri laboratories at the interface of physics and biology with complementary skills: the LMA with an expertise in ultrasonic imaging and neurostimulation, the IBDM in molecular biology of mechanosensory neurons and the Fresnel Institute in optical imaging of neuronal activity. The LMA (Franceschini group) is developing experimental approaches and applying physical concepts to achieve a quantitative understanding of physical mechanisms of US-induced neurostimulation. The IBDM (Moqrich group) is expert in the functional significance of primary sensory neurons in chronic pain, who will contribute to the understanding of molecular mechanisms and of sensory pathways triggered by US stimuli. The Fresnel institute (Rigneault – Chaigne group) is developing advanced optical neuroimaging techniques, enabling in vivo monitoring of neuronal activity by using multi-photon microscopy and/or photoacoustics.
Expected profile
The PhD candidate should hold a MSc (or equivalent degree) in physics, biophysics, acoustics, molecular or cellular biology, neurosciences or a related discipline. We aim to find a highly-motivated student with creative skills and appeal for experimental work. Ability to work in an interdisciplinary environment involving several research teams will be required. Some good English language skills and profusion in data analysis using, e.g. Matlab, will be a strong plus.
Is this project the continuation of an existing project or an entirely new one? In the case of an existing project, please explain the links between the two projects
During the PhD of Elena Brunet (IBDM-LMA Centuri 2020-24) and the ANR HearUS 2020-24, the US-sensitive
subpopulations of DRG neurons have been identified by combining primary neuron culture, calcium imaging and
single-cell RNA sequencing. By comparing our transcriptomic data with those already present in the databases,
the low-threshold C fibers have been identified as one of the most ultrasound-sensitive group. The aim of the
present Centuri PhD project aims to pursue this research to stimulate in vivo the low-threshold C fibers for
chronic pain treatment, adding a new in vivo neuro imaging component with the Fresnel laboratory.
2 to 5 references related to the project
- Hoffmann et al., Focused ultrasound excites action potentials in mammalian peripheral neurons in part
through the mechanically gated ion channel PIEZO2, PNAS, 119(21) 2022 (doi:
10.1073/pnas.2115821119) - Yoo et al., Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and
ion channel amplification, Nat Comm, 13:493 2022 (doi: 0.1038/s41467-022-28040-1) - Mittmann, Wolfgang, et al. "Two-photon calcium imaging of evoked activity from L5 somatosensory
neurons in vivo." Nature neuroscience 14.8 (2011): 1089-1093.
Two main publications from each PI over the last 5 years
- Merlo A., Losserand S., Yaya F., Connes C., Faivre M., Lorthois S., Minetti C., Nader E., Podgorski T.,
Renoux C., Coupier G., Franceschini E., “Influence of storage conditions and buffer composition on the
mechanical behaviour of flowing red blood cells”, Biophysical journal, 122 360-373, 2023 - Lombard O., Rouyer J., Debieu E., Blanc F., Franceschini E., “Ultrasonic backscattering and
microstructure in sheared concentrated suspensions”, J. Acoust. Soc. Amer. 147(3) 1359-1367, 2020 - Yoo S., Santos C., Reynders A., Marics I., Malapert P., Gaillard S., Charron A., Ugolini S., Rossignol R., El
Khallouqi A., et al. “TAFA4 relieves injury-induced mechanical hypersensitivity through LDL receptors
and modulation of spinal A-type K(+) current”. Cell Rep 37, 109884, 2021 - Hoeffel G., Debroas G., Roger A., Rossignol R., Gouilly J., Laprie C., Chasson L., Barbon P.V., Balsamo A.,
Reynders A., et al. “Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions”.
Nature 594, 94-99, 2021 - Saucourt J., Moreau A., Lumeau J., Rigneault H., Chaigne T., “Fast interrogation wavelength tuning for
all-optical photoacoustic imaging,” Opt. Express, OE, vol. 31, no. 7, pp. 11164–11172, Mar. 2023 - Schulze L., Henninger J., Kadobianskyi M., Chaigne T., Faustino A. I., Hakiy N., ... & Judkewitz B.,
“Transparent Danionella translucida as a genetically tractable vertebrate brain model”. Nature
methods, 15(11), 977-983, 2018
Project's illustrating image