PHD2024-08
Interplay of cellular forces, adhesion and mechanics during colorectal cancer progression
Host laboratory and collaborators
Elsa Bazellières, IBDM elsa.bazellieres@univ-amu.fr
Claire Valotteau, Dynamo claire.valotteau@inserm.fr
Marie Josée Santoni, Dynamo marie-josee.santoni@inserm.fr
Abstract
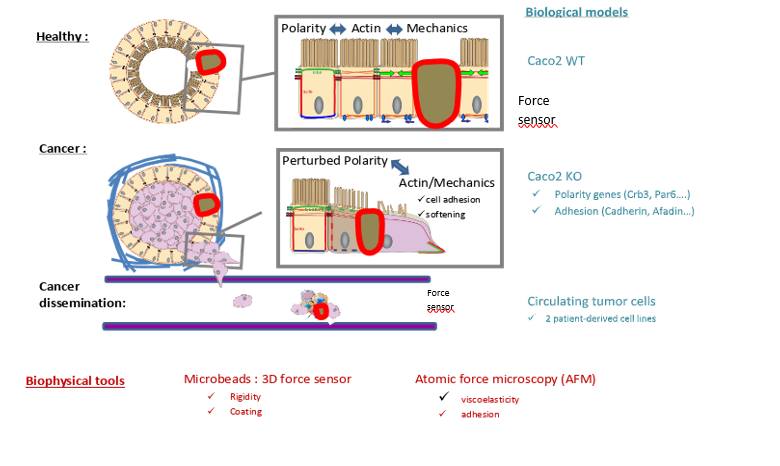
Intestinal epithelial tissues are 3D layered structures that display a high degree of organization and characteristic cell shapes. These properties rely on a complex interplay between cell polarity, junctional remodeling, and the actin cytoskeleton dynamics that regulate the monolayer mechanical properties. During cancer progression, epithelial tissues undergo several changes. The surrounding extracellular matrix stiffens, while cancer cells loosen their intercellular adhesions and soften. How exactly intrinsic cellular forces and adhesion are coupled to cancer cell mechanics adaptation remains elusive. Within this project, we will study how colorectal epithelia with different genetic backgrounds adapt their epithelial organization, stiffnesses and forces in 3D model systems. These results will be compared with observations made on circulating tumor cells derived from patient with colorectal cancer, mimicking a more advanced stage of cancer progression.
Keywords
Cell mechanics, adhesion, force, intestinal epithelia, colorectal cancer, cancer progression
Objectives
Hydrogel beads will be used to (i) investigate the mechanisms coupling cellular force generation to cell-cell adhesion strength, and (ii) study how these mechanisms are related to the evolution of cells stiffness during cancer progression.
Proposed approach (experimental / theoretical / computational)
Previously established and validated colorectal cellular models will be used. Human CaCo2 cells wild type and KO for cadherin or afadin have been generated (E. Bazellières, IBDM), and CTC44 and CTC45, two circulating tumor cells lines derived from colorectal cancer patients, are available (C. Valotteau, DyNaMo). CaCo2 cells form healthy 3D intestinal cysts, while patient derived circulating tumor cells exhibit tumor (CTC44) and metastatic (CTC45) phenotype. The mechanical properties and adhesion forces of these models will be measured by Atomic Force Microscopy (AFM, C. Valotteau, DyNaMo), while the forces generated by the cells inside the aggregates will be measured with deformable hydrogel beads (E. Bazellières, IBDM). Beads will be coated with different adhesion proteins (cadherins, nectins, occludins) to study the influence of these cellular adhesion entities on cell mechanics and bead rigidity will be tuned to mimic the overall adhesion and mechanics of the surrounding cells. Actin and/or cadherins dynamics together with bead deformation will be imaged on a spinning disk confocal microscope. From the bead deformation, cellular forces will be extracted using computational inversion methods previously developed in collaboration with Matthias Merkel (CPT).
Interdisciplinarity
The association between these 2 teams will bring together a strong expertise to address epithelial tissue organization, force regulation and mechanical adaptation during tumor progression. EB has developed the force sensors, the biological tools and has the expertise in live imaging and data analysis, while CV has a strong experience in both cell mechanics and cell adhesion characterization.
Expected profile
The PhD student must have a strong interest in collaborative and interdisciplinary research. Ideally, he/she should have some basic experimental skills (cell culture, imaging). Though programming skills are not a must, they are a clear advantage. An interest in dealing with theoretical concepts is expected. The candidate will be given the opportunity to learn state-of-the-art experimental techniques (CRISPR/Cas9, microgels, AFM), image analysis techniques (image processing, mathematical representation of objects), and physical concepts (tensors, elasticity theory, spherical harmonics). Overall, the student will both develop the skills required to successfully carry out the project, and acquire a versatile and solid set of tools that will be useful for interdisciplinary biophysical research.
Is this project the continuation of an existing project or an entirely new one? In the case of an existing project, please explain the links between the two projects
This project is a new collaboration between E. Bazellières and C. Valotteau, which relies on the expertise in in situ force measurement within 3D epithelial tissues and AFM characterizations. E. Bazellières has implemented small force sensors to measure forces in 3D epithelial systems with ECM molecules coated hydrogel beads. This project will complement a running project at DyNaMo, in which the biophysical properties of circulating tumor cells and clusters are studied using acoustic force spectroscopy in the context of an ATIP avenir grant.
2 to 5 references related to the project
- Gensbittel, V., Kräter, M., Harlepp, S., Busnelli, I., Guck, J., & Goetz, J. G. (2021). Mechanical adaptability of tumor cells in metastasis. Developmental cell, 56(2), 164-179.
- Au, S. H., Storey, B. D., Moore, J. C., Tang, Q., Chen, Y. L., Javaid, S., et al. (2016). Clusters of circulating tumor cells traverse capillary-sized vessels. Proceedings of the National Academy of Sciences, 113(18), 4947-4952.
- N. Träber, K. Uhlmann, S. Girardo, G. Kesavan, K. Wagner, J. Friedrichs, R. Goswami, K. Bai, M. Brand, C. Werner, D. Balzani & J. Guck (2019). Polyacrylamide Bead Sensors for in vivo Quantifcation of Cell-Scale Stress in Zebrafsh Development. Scientific Reports 9:17031
- Valentina Palmieri, Donatella Lucchetti, Alessandro Maiorana, Massimiliano Papi, Giuseppe Maulucci, Gabriele Ciasca, Maria Svelto, Marco De Spirito, and Alessandro Sgambato. Biomechanical investigation of colorectal cancer cells. Applied Physics Letters 105, 123701 (2014)
Two main publications from each PI over the last 5 years
- Bazellieres E, Le Bivic André. Mechanoregulation of PDZ Proteins, An Emerging function, Methods Mol Biol (2021) 2256:257-27
- Le Borgne-Rochet M, Angevin L, Bazellières E, Comunale F, Denisov EV, Tashireva LA, Perelmuter VM, Bièche I, Vacher S, Plutoni C, Bodin S, Gauthier-Rouvière C, P-cadherin-induced collagen fiber alignment is required for directional collective cell migration, J Cell Sci (2019) 132(21).
- Eroles M, Lopez Alonso J, Ortega A, Boudier T, Gharzeddine K, Lafont F, Franz C, Millet A, Valotteau C, Rico F, Coupled mechanical mapping and interference contrast microscopy reveal viscoelastic and adhesion hallmarks of monocyte differentiation into macrophages, Nanoscale (2023) 15.
- Valotteau C., Sumbul F., Rico F. High-speed force spectroscopy: microsecond force measurements using ultrashort cantilevers. Biophysical Reviews (2019), 11 (5), 689-699.
Project's illustrating image