PHD2024-05
SHINY: Emergence of a large-scale multicellular actin star network for epithelial connectivity and differentiation
Host laboratory and collaborators
DELACOUR Delphine, IBDM delphine.delacour@univ-amu.fr
RUPPRECHT Jean-François, CPT rupprecht@cpt.univ-mrs.fr
Abstract
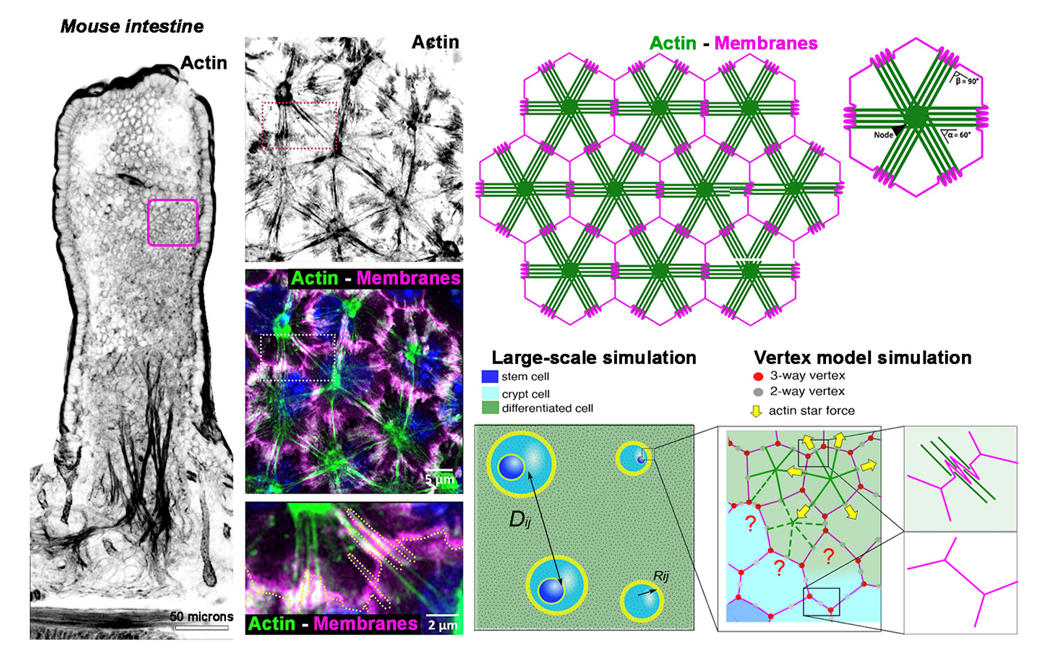
Actin structures have been shown to play a crucial role in tissue morphogenesis. The mouse intestinal epithelial tissue displays actin stars (AcSs), assembled in a large-scale supra-cellular network connecting differentiated cells through the middle of cell-cell junctions. AcSs can form in a large set of epithelial tissues, both in vivo and in vitro, and are conspicuously absent from the proliferative crypt compartment. The structured multicellular architecture of the AcS network may act as a "crystalline" framework, preserving the organization of cells within the differentiated domain in the intestinal epithelium. Here, mouse intestinal organoid tools, with standardized culture techniques and advanced genetic methods combined with live imaging, will be used in combination with biophysical analyses and physical modeling to test models of the possible mechanical role that the AcS network plays at various scales for tissue morphogenesis.
Keywords
Tissue morphogenesis, pattern formation, cytoskeleton, cell junctions, cell connectivity, physics of biological systems, vertex model, deep-learning-based joint segmentation, tracking tools
Objectives
The AcS cytoskeletal assembly has only been observed in vitro within purified reconstituted minimal actin systems (1,2), and reported only once in vivo within a sponge model, tentatively referred to as a 'histoskeleton' in the 1980s, although no specific function was attributed to it (3). Given the novelty of these observations and our complementary methodologies, we wish to explore the interplay between the extensive AcS network and tissue morphogenesis. We hypothesize that the AcS network ensures morphological and functional stability upon the epithelium at various scales, individual epithelial cells and epithelial assemblies and ultimately impact the tissue patterning.
Proposed approach (experimental / theoretical / computational)
In parallel to the in vivo mouse intestine model, we use potent intestinal organoid tools developed in the lab, with standardized culture techniques and advanced genetic methods combined with live imaging to unravel cellular mechanisms governing epithelial homeostasis (4). Here, we integrate these advancements with high-resolution cell and developmental biology (DD’ team), along with biophysical analyses and physical modeling (JFR’ team; 5) to decipher the functions of AcS:
- at the individual cell level for cell shaping and differentiation, and at the collective level for restricting cell intercalation and migration events. We will design a joint cell segmentation and cell classifier (crypt versus differentiated cells), together with an automatic detection code for cell divisions and cell rearrangements, notably deep-learning-based, joint segmentation and tracking tools;
- at the tissue level for epithelial compartment self-organization and stabilization. Quantitative analyses by segmenting tissue domains and using a cell-based simulation code will be used to test models of the possible mechanical role that the AcS network plays and its effects on tissue-level coarsening kinetics.
Interdisciplinarity
The project is interdisciplinary at its core, at the crossroads of biology, physics and computer science: indeed, deep learning-based tools will help us recast the problem of cell differentiation in mechanics concepts. The PIs have complementary expertise in those fields: the lead supervisor, D. Delacour, a CNRS developmental cell biologist, group leader at IBDM Marseille, focuses on understanding epithelial morphogenesis, homeostasis, and intestinal pathologies, with expertise in cell dynamics, particularly in 2D and 3D model systems. In 2017, she was awarded the Research Prize on Rare Intestinal Diseases from the Foundation Groupama. In June 2023, she established a new team dedicated to mammalian epithelial morphogenesis at IBDM in Marseille, equipped with animal housing and advanced imaging facilities. The co-supervisor, JF. Rupprecht, a CNRS theoretical physicist and group leader at the Centre de Physique Théorique/CENTURI Marseille, specializes in vertex models for tissues, mechano-hydrodynamic models for actin cortex, and image analysis techniques, including automated detection tools. Both partners have obtained solid results, yet unpublished, providing a strong foundation for the project.
Expected profile
The project blends different aspects, both experimental and theoretical, corresponding to a wide range of profiles. We are looking for profiles ranging from (1) someone who enjoys performing experiments and developing image analysis, while performing some simulations, to (2) someone who enjoys performing the image analysis and simulations, and some experiments. In any case, interdisciplinary will be key to the project. We target motivated and talented in either Biology or Physics students.
Is this project the continuation of an existing project or an entirely new one? In the case of an existing project, please explain the links between the two projects
It is a new project.
2 to 5 references related to the project
- Murrell MP and Gardel ML (2012). F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc Natl Acad Sci U S A 109, 20820-20825
- Vogel SK, Heinemann F, Chwastek G and Schwille P (2013). The design of MACs (minimal actin cortices). Cytoskeleton (Hobokn) 70, 706-717
- De Ceccatty MP (1986). Cytoskeletal organization and tissue patterns of epithelia in the sponge E. mulleri. J Morphol 189, 45-65
- Saleh J, Fardin MA, Barai A, …, Minc N and Delacour D (2023). Length limitation of astral microtubules orients cell divisions in murine intestinal crypts. Dev Cell 58, 1519-1533 e1516
- Nishizawa K, Lin SZ, Chardes C, Rupprecht JF and Lenne PF (2023). Two-point optical manipulation reveals mechanosensitive remodeling of cell-cell contacts in vivo. Proc Natl Acad Sci U S A 120, e2212389120
Two main publications from each PI over the last 5 years
Delacour:
- Gaston C, De Beco S, Doss B, …, Lim CT, Ladoux B and Delacour D (2021). EpCAM promotes endosomal modulation of the cortical RhoA zone for epithelial organization. Nat Commun 12, 2226
- Saleh J, Fardin MA, Barai A, …, Minc N* and Delacour D* (2023). Length limitation of astral microtubules orients cell divisions in murine intestinal crypts. Dev Cell 58, 1519-1533 e1516
J.F. Rupprecht:
- Prasad, N. Obana, S.-Z. Lin, K. Sakai, C. Blanch-Mercader, J. Prost, N. Nomura, J.-F. Rupprecht*, J. Fattaccioli*, A. S. Utada*, Alcanivorax borkumensis Biofilms Enhance Oil Degradation By Interfacial Tubulation, Science (2023)
- Nishizawa, S.-Z. Lin, C. Chardes, J.-F. Rupprecht*, P.-F. Lenne*, Two-point optical manipulation reveals mechanosensitive remodeling of cell-cell contacts in vivo, PNAS (2023)
Project's illustrating image