PHD2024-01
Social coding in the mouse cortex
Host laboratory and collaborators
Antoine de Chevigny, INMED antoine.de-chevigny@inserm.fr
Hervé Rouault, CPT herve.rouault@univ-amu.fr
Abstract
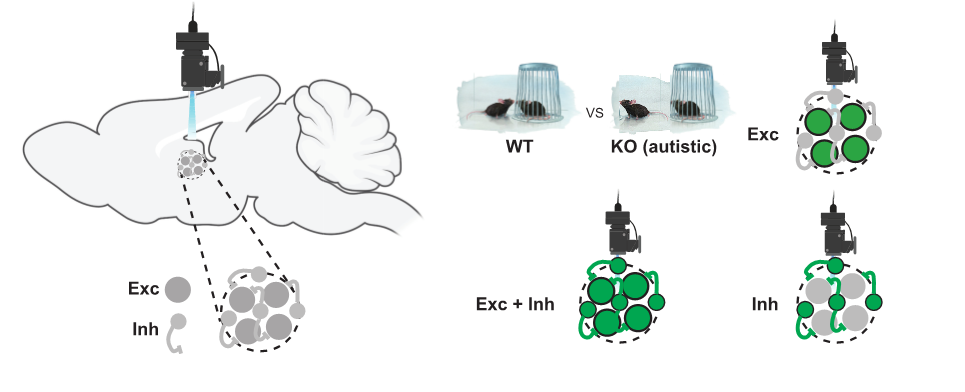
The objective of the project is to study how social interactions and memories are encoded in the mouse prefrontal cortex. We recorded the activity of a set of neurons by INSCOPIX microendoscopy calcium imaging while the mouse could freely investigate familiar or novel conspecifics in a social interaction arena. We are now in the process of using cutting-edge supervised and non-supervised machine learning techniques to related the measured neuronal activity with the social behavior of the animals. We would like to assess the performance of these methods and compare them with results obtained on other brain areas. These computational pipelines will then be used to characterize the neuronal activity change in a mouse model for autism (Neurod2 mutation).
The project requires experience in machine learning and signal analysis techniques.
Keywords
Social coding — Autism — Calcium imaging — Machine learning — Signal analysis
Objectives
The project aims at determining how neurons in the cortex encode social behaviors such as investigation and familiarization at single cell and cell population levels, in normal vs autistic conditions.
Proposed approach (experimental / theoretical / computational)
We recorded the calcium activity of a set of neurons by INSCOPIX microendoscopy while the mouse could freely investigate familiar or novel conspecifics in a social interaction arena.
We will design analysis pipelines with the help of modern machine learning toolbox (scikit-learn, PyTorch). We will then use theoretical tools to characterize the geometry of the neuronal activity space, determine a coding scheme and elaborate on its function.
Having recorded mice that carried Neurod2 mutation (an autism model), we will test how coding scheme and social behavior interplay.
Interdisciplinarity
The student will work in close collaboration with a wet lab post-doc who is performing all miniscope surgeries and behavior experiments.
Expected profile
We are looking for a physicist or a computer scientist with experience or strong interest in machine learning techniques and theory. A further interest in neuroscience would be greatly appreciated.
Is this project the continuation of an existing project or an entirely new one? In the case of an existing project, please explain the links between the two projects
The project started 2 years ago. Given the technical complexity of INSCOPIX experiment implementation, we only had the time to perform wet lab experiments (recordings of social interactions while filming brain activity). We now need to analyze the complex datasets (and possibly suggest new wet lab experiments).
2 to 5 references related to the project
- Phillips ML, Robinson HA, Pozzo-Miller L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife. 2019 May 21;8:e44182.
- Xing B, Mack NR, Guo KM, Zhang YX, Ramirez B, Yang SS, Lin L, Wang DV, Li YC, Gao WJ. A Subpopulation of Prefrontal Cortical Neurons Is Required for Social Memory. Biol Psychiatry. 2021 Mar 1;89(5):521-531.
- Park G, Ryu C, Kim S, Jeong SJ, Koo JW, Lee YS, Kim SJ. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell Rep. 2021 May 11;35(6):109104.
- Xu P, Yue Y, Su J, Sun X, Du H, Liu Z, Simha R, Zhou J, Zeng C, Lu H. Pattern decorrelation in the mouse medial prefrontal cortex enables social preference and requires MeCP2. Nat Commun. 2022 Jul 6;13(1):3899.
- Boyle L et al., The geometry of hippocampal CA2 representations enables abstract coding of social familiarity and identity. Biorxiv 2023
Two main publications from each PI over the last 5 years
Antoine de Chevigny
- Faure L et al. Single cell RNA sequencing identifies early diversity of sensory neurons forming via bi-potential intermediates. Nat Commun. 2020 Aug 21;11(1):4175.
- Runge K et al. Disruption of NEUROD2 causes a neurodevelopmental syndrome with autistic features via cell-autonomous defects in forebrain glutamatergic neurons. Mol Psychiatry. 2021 Nov;26(11):6125-6148.
Hervé Rouault
- Modi MN et al. Flexible specificity of memory in Drosophila depends on a comparison between choices. Elife. 2023 Jun 15;12:e80923.
- Dard RF et al. The rapid developmental rise of somatic inhibition disengages hippocampal dynamics from self-motion. Elife. 2022 Jul 20;11:e78116.
Project's illustrating image