PHD2024-21
Deep-tissue photoacoustic imaging of calcium activity in rodents and non-human primates
Host laboratory and collaborators
Thomas CHAIGNE, Institut FRESNEL thomas.chaigne@fresnel.fr
Jean-Claude Platel, Inmed jean-claude.platel@inserm.fr
Guillaume MASSON, Institut des Neurosciences de la Timone guillaume.masson@univ-amu.fr
Ivo VANZETTA, Institut des Neurosciences de la Timone ivo.vanzetta@univ-amu.fr
Abstract
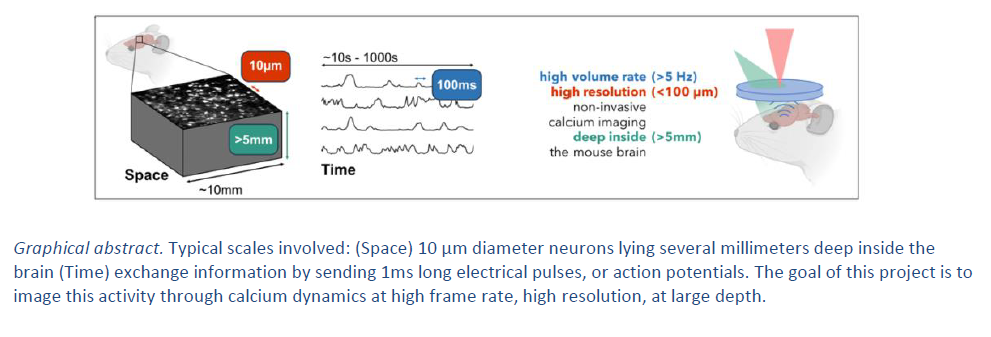
Measuring the activity of neurons in vivo is crucial for understanding brain functions. Current optical micro- and meso-scopy enables activity recordings using fluorescent calcium-sensitive indicators. However, due to light scattering, its maximal accessible depth is less than a millimeter. We want to adapt photoacoustic imaging, a technique capable of measuring optical absorption several millimeters deep in tissue, to the recording of neuronal activity with (near-)cellular resolution. It has recently been shown that fluorescent calcium indicators generate photoacoustic signals, as these molecules are optical absorbers. However, previous works used low-frequency piezoelectric sensors, which limit spatial resolution to about 200 μm. We propose to use an all-optical photoacoustic imaging system that can optically detects ultrasound and thus provides higher resolution (~50 μm). This method can acquire 3D volumes at speeds compatible with the recording of functional signals. If successful, this development will allow, for the first time, imaging calcium activity non-invasively in deep structures such as hippocampus and subcortical nuclei, as well as in the intact spinal cord, all the way to the central canal.
Keywords
Photoacoustics, optical neuro-imaging, molecular calcium probes
Objectives
The goal of this project is to image neuronal activity in vivo in rodents and non-human primates, at a depth of several millimeters, with a resolution of 50 μm, in the intact brain and the spinal cord. The first step is to characterize the system in brain or spinal cord slices in vitro, by simultaneously measuring photoacoustic signals, fluorescence, and local field potentials. We shall then move to the recording of neural activity in-vivo, in order to study selectively the activity in different cortical layers, and, as non-invasively as possible, that of cerebro-spinal fluid-contacting neurons in the central canal.
Proposed approach (experimental / theoretical / computational)
First, we will characterize the exact relationship between the photoacoustic signal from calcium indicators and the electrical activity of neurons. We will therefore build a multi-modal setup combining a photoacoustic imaging system, a widefield epi-fluorescence microscope and a multi-electrode array chip. Following this first in vitro characterization, we will develop an in vivo multispectral photoacoustic imaging scheme to separate calcium sensors from blood vessels, which will provide large background photoacoustic signals. To ensure sufficient resolution, we will develop a protocol to install polymer-based cranial windows compatible with ultrasound propagation. This should maximize the high frequency content of the detected photoacoustic signals, and therefore the resolution. Thanks to these technological developments, we will be able to look into the deeper regions of the central nervous system in a non-invasive manner, to selectively study neuronal activity in vivo in different cortical layers and in the hippocampus. Experiments aimed to record the activity of cerebro-spinal fluid-contacting neurons in the spinal cord’s central canal will first be performed in-toto, aiming for in-vivo only at a later stage.
Interdisciplinarity
This collaborative project is articulated around an all-optical photoacoustic imaging system, which has been installed on the recent Circuitphotonics imaging facility, established jointly by three Centuri laboratories: Inmed, the INT and the Fresnel Institute. This collaborative effort in general, and the development of photoacoustic imaging for calcium indicators in particular, will require a comprehensive approach across multiple fields. It will therefore involve expertises in physics, optics, electrical engineering, image and signal processing, as well as neurosciences and, to some extent, molecular biology. This challenge will be addressed by joining several experts from the three Centuri partner labs (Fresnel Institute, INT, Inmed), to provide the required know-how to fully harness the capabilities of photoacoustic imaging for neuroscientific applications. The Fresnel Institute (Chaigne group) is developing advanced all-optical photoacoustic techniques to image non-invasively deep regions in the brain. The Inmed (Cossart group) will contribute with its expertise in hippocampal imaging and INT with its expertise in cortical imaging in rodents (Vanzetta group) and non-human primates (Masson group).
Expected profile
The PhD candidate should hold a MSc (or equivalent degree) in physics, optics, acoustics, electrical engineering, neurosciences or any related discipline. We aim to find a highly-motivated student with creative skills and appeal for experimental work. Ability to work in an interdisciplinary environment involving several research teams will be required. Basic programming skills (Matlab or Python) are essential, as well as a certain taste for tinkering. As he/she will be evolving in an international environment, the candidate must be fluent in English (at least C1), and exhibit excellent communications capabilities (written and spoken).
Is this project the continuation of an existing project or an entirely new one? In the case of an existing project, please explain the links between the two projects
The collaboration has been already established, in particular in the context of the new Circuitphotonics facility that has been recently established in Marseille between Fresnel Institute, INT, Inmed. It has received some dedicated funding on top the initial Equipex+, from the Marseille Imaging Institute (IMI), and already generated some preliminary data. A Master 2 student (funded by IMI) will work on the same subject from January to June 2024. The recruitment of a dedicated PhD student is therefore both timely and essential to foster this recent and dynamic collaboration.
2 to 5 references related to the project
- P. Beard, “Biomedical photoacoustic imaging,” Interface Focus, vol. 1, no. 4, pp. 602–631, Aug. 2011, doi:10.1098/rsfs.2011.0028.
- E. Zhang, J. Laufer, and P. Beard, “Backward-mode multiwavelength photoacoustic scanner using a planar Fabry-Perotpolymer film ultrasound sensor for high-resolution three-dimensional imaging of biological tissues,” Appl. Opt., AO, vol.47, no. 4, pp. 561–577, Feb. 2008, doi: 10.1364/AO.47.000561.
- S. V. Ovsepian, I. Olefir, G. Westmeyer, D. Razansky, and V. Ntziachristos, “Pushing the Boundaries of Neuroimagingwith Optoacoustics,” Neuron, vol. 96, no. 5, pp. 966–988, Dec. 2017, doi: 10.1016/j.neuron.2017.10.022.
- X. L. Deán-Ben et al., “Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calciumindicators,” Light: Science & Applications, vol. 5, no. 12, p. e16201, Aug. 2016, doi: 10.1038/lsa.2016.201.
Two main publications from each PI over the last 5 years
Thomas Chaigne
- J. Saucourt, A. Moreau, J. Lumeau, H. Rigneault, and T. Chaigne, “Fast interrogation wavelength tuning for all-opticalphotoacoustic imaging,” Opt. Express, OE, vol. 31, no. 7, pp. 11164–11172, Mar. 2023
- Schulze, L., Henninger, J., Kadobianskyi, M., Chaigne, T., Faustino, A. I., Hakiy, N., ... & Judkewitz, B. TransparentDanionella translucida as a genetically tractable vertebrate brain model. Nature methods, 15(11), 977-983. (2018)
Jean Claude Platel
- Bollmann Y, Modol L, Tressard T, et al. Prominent in vivo influence of single interneurons in the developing barrelcortex. Nat Neurosci., 26(9):1555-1565. doi:10.1038/s41593-023-01405-5 (2023).
- Bugeon S, Haubold C, Ryzynski A, Cremer H, Platel JC. Intrinsic Neuronal Activity during Migration Controls theRecruitment of Specific Interneuron Subtypes in the Postnatal Mouse Olfactory Bulb. J Neurosci., 41(12):2630-2644.doi:10.1523/JNEUROSCI.1960-20.2021 (2021).
Guillaume Masson
- Meso, A.I., Gekas, N., Mamassian, P. and Masson, G.S., 2022. Speed estimation for visual tracking emerges dynamicallyfrom nonlinear frequency interactions. Eneuro, 9(3).
- Benvenuti, G., Chemla, S., Boonman, A., Perrinet, L., Masson, G.S. and Chavane, F., 2020. Anticipatory responses alongmotion trajectories in awake monkey area V1. BioRxiv, pp.2020-03.
Ivo Vanzetta
- Kaszas A, Szalay G, Slézia A, Bojdán A, Vanzetta I, Hangya B, Rózsa B, O'Connor R, Moreau D. Two-photon GCaMP6fimaging of infrared neural stimulation evoked calcium signals in mouse cortical neurons in vivo. Scientific Reports. 11:9775. DOI: 10.1038/s41598-021-89163-x (2021).
- Donahue MJ, Kaszas A, Turi GF, Rózsa B, Slézia A, Vanzetta I, Katona G, Bernard C, Malliaras GG, Williamson A.Multimodal Characterization of Neural Networks Using Highly Transparent Electrode Arrays. Eneuro. 5. DOI:10.1523/ENEURO.0187-18 (2018).
Project's illustrating image